Theranostics, combining therapy and diagnosis, has revolutionized prostate cancer treatment by using PSMA-targeted radiopharmaceuticals for precise imaging and customized therapy, significantly improving patient outcomes.

Theranostics is a giant leap forward in nuclear medicine. What is theranostics? Theranostics simply means combining therapy and diagnosis.
Theranostics has rapidly developed into a viable option for the simultaneous implementation of cancer diagnostics and customized treatment (precision oncology). Theranostics is based on labeling the same molecule with both a diagnostic (most often positron-emitting) and a therapeutic (beta-emitting) isotope. In this case, the treatment dose can be tailored exactly according to the patient, and the actual treatment dose can also be imaged.
Of course, this concept is not new to nuclear medicine. Rather, it has been used for decades to treat thyroid diseases; the first radioiodine therapy was given in the 1940s. In recent years, however, theranostics have been successfully used for a whole range of other tumor diseases. These primarily include neuroendocrine tumors and prostate cancer. The effectiveness of the therapy of neuroendocrine tumors with radioactively labeled analogues of the peptide hormone somatostatin was definitely demonstrated in the NETTER-1 registration trial, leading to the approval of the ligand 177Lu-DOTA-TATE (LutaThera®) in Europe and the USA.
Theranostics (or theragnostics) refers to a close connection between diagnostics and the resulting therapy. In principle, any combination of diagnostics and therapy can be used, e.g. a laboratory value and the resulting consequences for drug therapy. However, the term theranostics is increasingly being used specifically for nuclear medicine imaging and therapy using the same or two very similar radiopharmaceuticals. In parallel, positron-labeled somatostatin analogues 68Ga-DOTA-TATE and 68Ga-DOTA-TOC were approved for neuroendocrine tumor imaging and patient selection for radionuclide therapy.
The VISION and TheraP trials aimed at approving a radioactively labeled PSMA ligand for the treatment of metastatic prostate cancer were completed in 2021. After these phase III studies on the diagnosis and treatment of prostate cancer using PET/CT and radioactively labeled PSMA ligands, the therapeutic compound 177Lu-PSMA-617 was approved by 2022 both in Europe and the USA.
The prostate-specific membrane antigen (PSMA) has recently been established as a predictive biomarker for the staging of prostate cancer, as the expression of PSMA correlates with the severity of the disease. At the same time, its extracellular domain (i.e. surface structures) offers an excellent target for the radiopharmaceutical development of PSMA-targeting inhibitors, which can be used for both diagnostic imaging and therapeutic purposes. The abbreviation PSMA stands for prostate-specific membrane antigen. Like PSA, it is a protein (an enzyme) that the body can produce itself and therefore a known substance to the human organism. The PSMA uptake also occurs on the surface of healthy prostate cells, but only in small quantities. Significantly more PSMA - around 1000 times more - can be detected on prostate cancer cells and metastases. The more aggressive a man's prostate cancer is (the higher the Gleason score), the greater the amounts of PSMA are on the cancer cells. In the rest of the body, however, the protein is almost non-existent.

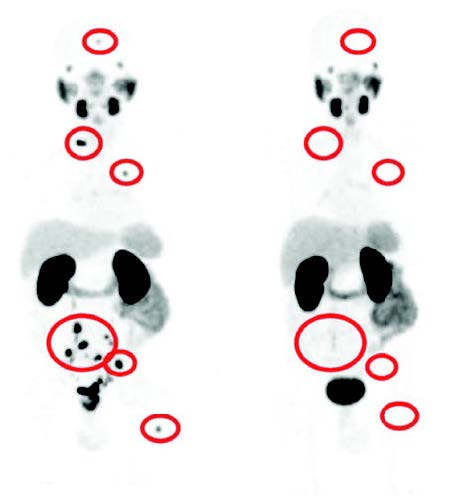
By using positron emission tomography (PET) method, nuclear medicine physicians can visualize regions in the body where metabolism is particularly active. This is the case with cancer cells, for example. They require a lot of energy because they divide and reproduce quickly. PET works with radioactively labeled drugs, called tracers or radionuclides. Examples are 68-gallium (68Ga) or 18-fluorine (18F). They are injected into the vein, distributed throughout the body via the bloodstream and attach specifically to the PSMA protein.
68Ga-PSMA-617 compounds have received FDA approval as well as a few 18F-PSMA-compounds. There is no practical difference in diagnostic accuracy between these tracers. The 68Ga-PSMA-617 tracers are used as commercial radiopharmaceuticals Illuccix® and Locametz® and 18F-PSMA-compounds are e.g. known as Posluma® and Pylarify®.
The abbreviation CT means computer tomography - an imaging procedure that uses X-rays. Radiologists break the body “into slices” and obtain high-resolution cross-sectional images of the inside of the body. CT is a standard procedure that is used in the diagnosis of many medical conditions.
For every PET/CT examination, radiologists carry out both a PET and a computer tomography (CT). There are now devices that can do both. The binding of the tracer to the PSMA can be made visible in this way. This makes it possible to distinguish very precisely between healthy and malignant cells in the prostate and to spatially assign the findings using CT.
PSMA-PET/CT allows very precise conclusions to be drawn about a relapse of prostate cancer and any metastases that may be present. Doctors use them, for example, when the PSA level increases inexplicably after cancer treatment, such as surgery, radiation, or hormone therapy. The method has greater accuracy in detecting prostate cancer metastases than, for example, the combination of computed tomography and bone scintigraphy.
The cancer patients are cared for and treated by teams of doctors from various disciplines, multidisciplinary teams. The diagnostic indications of using PSMA-PET/CT may vary locally.
PSMA-PET/CT is suitable for diagnosing the spread of men with a Gleason score from 8 to 10, a cancer stage of T3 or T4, or a serum PSA value greater than 20 µg/l. At these values, the risk of metastases is very high. Therefore, these men are now entitled to a PSMA-PET/CT right from the start to determine the spread of the prostate cancer. But from wide international experience it is known, the much lower values give positive imaging results. Both 68Ga- and 177Lu-PSMA-compounds are available for diagnosis and therapy in Canada.
Author:
Kalevi Kairemo, MD, PhD, MSc(Eng)
Professor, Consultant in Nuclear Medicine,
Clinical Chemistry, Pharmaceutical Medicine & Health Care Administration
President 2024-5, World Association of Radiopharmaceutical and Molecular Therapy (WARMTH)