When cancer has spread within the body the 2 main pillars of treatment are drugs such as chemotherapy which are administered internally and distribute throughout the body, and radiation which delivers tumour-destroying radiation from outside the body. However there is another approach...
A GAME CHANGER CONCEPT
When cancer has spread within the body the 2 main pillars of treatment are drugs such as chemotherapy which are administered internally and distribute throughout the body, and radiation which delivers tumour-destroying radiation from outside the body. However there is another approach, in essence a hybrid of these 2, which is making a big impact in the management of neuroendocrine tumours (NETs) and is poised to improve the management of other cancers. LUTATHERA® lutetium (177Lu) oxodotreotide is a “radioisotope therapy”, in which a tumour-seeking molecule (in this case DOTATATE) is attached to a radiation-emitting isotope (in this case 177Lutetium (177Lu)). LUTATHERA® lutetium (177Lu) oxodotreotide circulates throughout the body, binding to tumour cells and emitting radioactivity that attacks the tumour cells. Hence even if the cancer has spread diffusely it is targeted by this form of radiation. Actually, the concept itself is not new. Radioactive Iodine has been used since the 1940’s to treat thyroid cancer. The thyroid gland – and cancers derived from it – capture Iodine. While the tumour-seeking properties of Iodine were known, the initiative to administer newly-developed radioactive isotopes of Iodine to thyroid cancer patients was visionary. Modern radioisotope therapies such as LUTATHERA® lutetium (177Lu) oxodotreotide apply advances in science to develop specific molecules that target other types of cancer. Most radioisotope therapies have an imaging counterpart which demonstrates where the therapy will be delivered, an important benefit which is unique among cancer therapies.
NEUROENDOCRINE TUMOURS: AT THE LEADING EDGE OF MODERN THERANOSTICS
Neuroendocrine tumours arise from specialized neuroendocrine cells present in multiple organs, including the small bowel, pancreas, lungs, and adrenal glands. Many will spread (“metastasize”) to other organs such as the liver, lymph nodes, or bone. A unique feature of NETs is that many produce – and in fact over produce – peptides and hormones that are normally produced in small quantities by the organs from which they arise. For example, NETs arising from the small bowel often produce serotonin which leads to the “carcinoid syndrome” (excessive diarrhea, flushing, asthma-like symptoms, and damage to heart valves) while NETs arising from the pancreas can produce deleterious amounts of insulin, gastrin, and other pancreatic hormones. Biologically, most NETs are low grade and do not respond well to conventional chemotherapies. However, most express large numbers of somatostatin receptors (SSRs) on their cell surface, and it is these receptors that are targeted by LUTATHERA® lutetium (177Lu) oxodotreotide: the molecule DOTATATE is a somatostatin analog and binds to SSRs. Following injection Lutetium (177Lu) oxodotreotide circulates throughout the body, binding in high concentrations to the tumour cells no matter how extensively they have spread. It emits radiation via the radioactive decay of the 177Lutetium which travels very short distances, attacking the DNA of the tumour cells. This approach is also known as Peptide Receptor Radionuclide Therapy (PRRT).
68Gallium-DOTATATE PET-CT Scan.
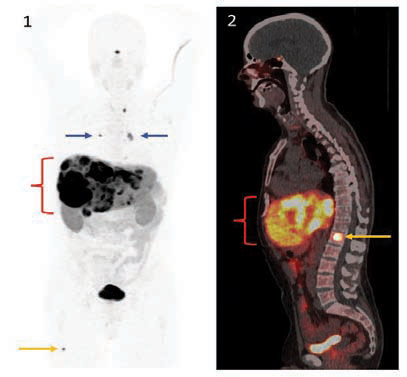
Figure 1: This overview (“MIP”) image from the PET scan demonstrates intense uptake of 68Gallium-DOTATATE in multiple liver metastases ( { ). Small lymph node metastases are also clearly detected (blue arrows), as well as a tiny metastasis to the femur bone (orange arrow).
Figure 2: Sagittal midline fused PET and CT images from the same scan provide both molecular (PET) and anatomic (CT) detail. In addition to the liver metastases, a metastasis is demonstrated in a spine vertebral body (orange arrow).
68Gallium-DOTATATE PET-CT scans detect sites of metastatic disease with high resolution. Importantly, they accurately depict exactly where the LUTATHERA® lutetium (177Lu) oxodotreotide therapy will go, as the tumor-seeking molecule is the same.
EFFECTIVENESS OF LUTATHERA® LUTETIUM (177LU) OXODOTREOTIDE
LUTATHERA® lutetium (177Lu) oxodotreotide is now the preferred therapy for patients with NETs progressing in spite of the standard treatment with a (non-radioactive) somatostatin analog provided certain conditions are met such as confirmation of targets on imaging, discussed below. A landmark phase 3 randomized control study, NETTER-1, clearly demonstrated that patients treated with 177Lu-DOTATATE had much better outcomes such as progression-free survival than those who did not receive it. This pivotal trial included only patients whose NETs were derived from the midgut (much of the small bowel and a portion of the large bowel, the most common NETs). Lutathera has a Health Canada approval for: The treatment of unresectable or metastatic, well-differentiated, somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumours (GEP-NETs) in adults with progressive disease.
However, funding in some jurisdictions, including Canada, at the time of this writing, Lutathera is only approved for NETs of mid gut origin. The treatment of NETs arising from the pancreas is approved in some jurisdictions (and is funded in the province of Quebec) and is currently under evaluation for rest of Canada.
4 separate treatments are performed, each 8 weeks apart. In conjunction with the LUTATHERA® lutetium (177Lu) oxodotreotide infusion patients receive a slow injection of an amino acid solution over 4 hours to protect the kidneys, so each treatment takes about a half a day. The treatment is generally very well tolerated by patients.
IMAGING THE THERAPY TARGETS
A unique and valuable aspect of radioisotope therapies such as LUTATHERA® lutetium (177Lu) oxodotreotide is a “theranostic” counterpart, the same tumour-seeking molecule attached to a different radioisotope which generates diagnostic images (theranostic = therapeutic+ diagnostic). In this case the imaging isotope is 68Gallium (68Ga). While 177Lu decays by emitting beta particles which attack the tumour, 68Ga decays by emitting positrons which are detected in a Positron Emission Tomography (PET) scanner. 68Ga-DOTATATE PET scans are the most effective way of detecting NETs, and are particularly impactful in evaluating a patient for LUTATHERA® lutetium (177Lu) oxodotreotide therapy. The incorporation of LUTATHERA® lutetium (177Lu) oxodotreotide into tumours depends on the concentration of somatostatin receptors on tumour cells, and this can be quite variable between patients and even between tumour sites within a given patient. Performing a 68Ga-DOTATATE PET scan demonstrates exactly where and how intensely the LUTATHERA® lutetium (177Lu) oxodotreotide therapy will distribute: see figures. This certainty of target delivery is unique in systemic cancer treatment.
WHAT DOES THE FUTURE HOLD?
The effectiveness of theranostics in NETs is likely just the tip of the iceberg, as theranostic pairs for other cancers are in various stages of development. In fact, use in prostate cancer is already underway. Prostate-Specific Membrane Antigen (PSMA) is a molecule that binds to prostate cancer cells. As with DOTATATE for NETs, PSMA is labelled with 68Ga for PET scanning and with 177Lu for therapy, proving very effective in both. Commercial versions of both have recently been approved by the US FDA and at the time of writing are under evaluation by Health Canada. As prostate cancer is the most common cancer in men the impact will be large.
New and expanded concepts in theranostics are also evolving including:
Modern theranostics, refined in the management of neuroendocrine tumours, is poised to make a major contribution to the care of cancer patients.